"Biomarkers show us the condition of nerve fibers after an accident"
To understand pain, you have to understand the spinal cord. This part of the central nervous system is considered the first switch point on the way to the brain. In the search for possible pain therapies, the spinal cord is thus becoming more and more the focus of attention. However, before this can be achieved, the cord must be better understood, especially in the case of injuries. Up to now, tests to check the progress of therapy have measured how mobile or touch-sensitive a person affected by a spinal cord injury is. The problem is that these methods are quite imprecise. So-called biomarkers could provide a more precise tool. Nikolaus Weiskopf explains how this works, how these biomarkers can advance research on spinal cord lesions, and how the nervous system can become unbalanced. He is head of the Neurophysics department and director at the MPI CBS.

Prof. Weiskopf, the spinal cord is regarded as central when it comes to pain. Why is so little known about it?
Of course, we already know a lot about the spinal cord, as a part of the central nervous system. But it is, like the brain itself, highly complex. To understand it better, we need to do non-invasive research on humans and that is very difficult as the spinal cord is only as thick as a thumb. With today's MRI methods, with a resolution of about one millimeter, we only have about 10 to 15 data points in cross section. In addition, the spinal cord and its cerebrospinal fluid are constantly in motion, due to breathing and the heartbeat, but also due to the small movements the test persons make with their head and neck, even when they try to lie still in the MRI. These are natural disruptive factors that worsen the image.
In our research in the Department of Neurophysics we do not focus on pain itself. Instead, we focus on better understanding the anatomy of the spinal cord. In cooperation with colleagues at Balgrist University Hospital in Zurich, we are studying patients with spinal cord injuries, among other things. This should, of course, help to eventually treat such injuries and the paralysis that accompanies them. But it’s also meant to understand the spinal cord as a whole. But until then, many intermediate steps are needed.
What do those steps look like?
You have to know, for example, whether the treatment methods used are effective at all. This requires key figures, so-called markers. At the moment, clinical behavioral tests are primarily used for this purpose. For example, they look at how well the person concerned can move again, how well he or she feels touch, or whether he or she is in pain. However, the problem is that these tests are relatively inaccurate. We are therefore looking at magnetic resonance imaging, MRI, and the much more direct biomarkers that indicate the condition of nerve fiber bundles after an accident. And not only at the site where the spinal cord is damaged, but also at others, for example in the brain. Incidentally, this is also a reason why people with spinal cord injuries often develop chronic pain, even after the acute injury has healed.
In which way?
Overall, very little is known about this phenomenon. Only this much: in spinal cord injury, more than just the essential pathways are damaged. A whole range of changes occur and the entire order of the central nervous system is disrupted. For example, if the balance of the central nervous system is disturbed, even normal sensory stimuli can lead to pain. In order to avoid such effects, therapies must also ensure that the regenerated neuronal connections are properly interconnected. Our clinical cooperation partners in Zurich are also working to better understand these mechanisms.
Back to biomarkers. Which ones do you use here?
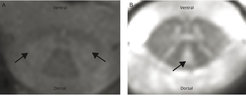
A very meaningful one is, for example, the high-resolution cross-section of the spinal cord. You can use this to measure the cross-sectional area. The more axons are damaged, i.e. the fibers, the smaller the cross-section is. Or, you can see from the cross-section which parts of the gray or white matter are affected, which then leads to different failures.
In addition to purely macroscopic anatomical markers, we also look at the microstructure of the tissue with the so-called quantitative MRI. This in turn is expressed approximately in the so-called longitudinal relaxation time, which indicates how long it takes for the spins in the tissue to return to their state of equilibrium after excitation. This in turn allows us to draw conclusions about structures that are smaller than an image element in the MRI. In particular, the fibers, the axons, which are less than one micrometer thick and surrounded by an insulating layer, the myelin sheath. Roughly speaking, the longer the longitudinal relaxation time, the thinner this myelin layer is and the more damaged the fibers are.
What have you found so far in this area?
In a recent study, published in Science Advances, we just found out that the superficial white matter in the brain contains a lot of iron. The iron is necessary to form the myelin sheath, the insulating layer around the fibers. So the iron content indirectly tells us something about fiber density and maturity. In a follow-up study, we now want to find out whether this also applies to the spinal cord. And we want to develop so-called in vivo markers, in which we use, for example, the mass of relative myelinization and the thickness of fibers. We are learning so much more about the importance of fibers. They play a crucial role, especially in the context of diseases and injuries, because this is where the tissue changes a lot.
We are currently working with Martin Schwab and Armin Curt from the University of Zurich, for example, to be able to heal spinal cord injuries at some point. By the way, Martin Schwab was the one who, about 30 years ago, found out why there are such serious limitations in the central nervous system when such injuries occur. If, on the other hand, you cut your finger, the nerve fibers usually grow back and after a certain time a feeling at the fingertip develops again. This does not happen in the central nervous system. Here, so-called no-go antibodies are formed, which prevent the nerve fibers from growing back. Schwab has in turn developed antibodies that are injected into the cerebrospinal fluid and are intended to neutralize these inhibiting no-go antibodies.
At the moment, this is in the second clinical test phase to determine the effectiveness of the treatment. Our quantitative MRI markers, such as the described cross-section of the spinal cord, are part of the study and should provide further information on efficacy. In recent years, we have also focused on establishing these new quantitative MRI methods in clinical research and, in the long term, in practice. This is often the problem: although there are great research methods that are used in specialized centers, they often reach patients very late.
If the spinal cord is so difficult to examine, how do you proceed?
In order to be able to measure the very fine structures and also functional activity signals in the spinal cord, we need a high so-called signal-to-noise ratio that allows us to clearly distinguish the actual measured values from the disturbing factors. For this purpose, we mainly use our new 7 Tesla MRI with its extremely strong magnetic field and special high-frequency coils that transmit and receive the radio waves. Our new coils come as close as possible to the spine, i.e. the spinal cord, so that they record more useful and less interfering signals. And we measure with up to 32 coils simultaneously. This in turn increases the ratio between useful and interfering signals and shortens the measurement time enormously. The test persons do not have to lie in the scanner for so long and the risk of moving is reduced.
Of course, we again have to contend with other challenges here, for example that the signals are received at different strengths depending on how close the section of the spinal cord is to the respective coil. So it is the many small, apparently quite practical steps we first have to take to eventually understand how the complex signal "pain" is related to the brain and spinal cord.
(The interview was conducted by Verena Müller.)




