First Experience with Arterial Blood Contrast (ABC) fMRI in the Human Motor Cortex at 7 Tesla
Measurements of brain function in humans is routinely performed using the Blood Oxygenation Level Dependent (BOLD) contrast (Kwong et al. 1992; Ogawa et al. 1992). In order to achieve maximum BOLD contrast, the time between the excitation of the spin system and the readout of the signal should be equal to the transversal relaxation time T2* of the tissue of interest. At 7 Tesla this time is in the order of 20 to 25 ms leading to long scan times given the latest progress in sparse sampling and imaging acceleration. Furthermore, the BOLD signal is biased towards larger veins and therefore limited in its spatial selectivity. During the last years, VASO (VAscular Space Occupancy) tackled the second issue by using cerebral blood volume as a marker for functional activity resulting in a better spatial correlation between neuronal activity and measured signal change (Huber et al. 2018). More recently another technique was developed associating the local increase in blood volume with brain activation -- mainly occurring in the arterioles and capillaries -- by utilizing magnetization transfer to suppress the signal differentially from grey matter relative to blood (Schulz et al. 2020; Priovoulos et al. 2023). Arterial blood contrast (ABC) is additive to the residual BOLD effect, but will have its maximum value at the time of excitation resulting in a better use of the acquisition time and therefore also tackling the first issue of BOLD contrast mentioned above.
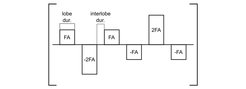
As a first step to implement ABC-fMRI at 7 Tesla we used a finger tapping (right hand) paradigm for activating the hand knob and measured combined ABC and residual BOLD contrast at different times post-excitation. We used a double-echo 3D-EPI sequence with 4 x 2 acceleration and 6/8 partial Fourier. The other parameters were set to: Repetition Time TR = 1920 ms; Echo Times TE1/2 = 11.8/27.8 ms; Receiver bandwidth BW = 1860 Hz/Px; 1.5 mm isotropic voxels. In an interleaved fashion, a Magnetization Transfer (MT) pulse (see Fig. 1) was and was not played out before acquisition while keeping the other parameters equal.
Activations maps for 5 subjects are shown in Figure 2, respectively. The left hand knob is activated in all cases. A decrease in the number of activation voxels is observable in images with MT preparation compared to images with MT off.

The corresponding signal time courses and mean activations in three different layers across the depth of motor cortex averaged over all 5 subjects are shown in Figure 3. The expected pattern of the hemodynamic response is apparent for all conditions. The overall signal is stronger at TE = 27.8 ms compared to 11.8 ms independent of MT preparation.

However, even at the lowest echo time achievable with the desired spatial resolution, a BOLD signal is present and the MT preparation does not have a significant effect on the signal amplitude. This can be explained by two challenges: First, the high field strength of 7 Tesla lead to a reduction in T2* compared to more conventional field strengths of 3 Tesla. Hence, the optimal echo time for the BOLD effect is lower in our experiment at 7 T than in the experiments by Schulz et al. (2020) performed at 3 T. Second, the lowest technically achievable echo time in our experiment (11.8 ms) is almost twice as long as the echo time used by Schulz et al. (6.9 ms). This is caused by the longer echo trains necessary for achieving a the higher spatial resolution (1.5 mm vs 3.0 mm isotropic) while maintaining a sufficient signal-to-noise ratio. Therefore, completely isolating the ABC contrast from the BOLD effect using high spatial resolution is challenging at 7 T. However, it could be shown that performing the experiment at 7 T is possible and its implementation may act as a future tool complementary to BOLD and VASO to investigate brain function.














