WP1 MPI CBS: In vivo qMRI imaging
Background
Prof. Dr. Nikolaus Weiskopf leads the Department of Neurophysics at the MPI CBS, Leipzig, Germany, which focuses on the development of novel methods for non-invasive MRI-based histology. This approach combines advanced MRI acquisition methods with biophysical models and multimodal atlases in order to glean information on tissue microstructure from macroscopically measured MR parameters.
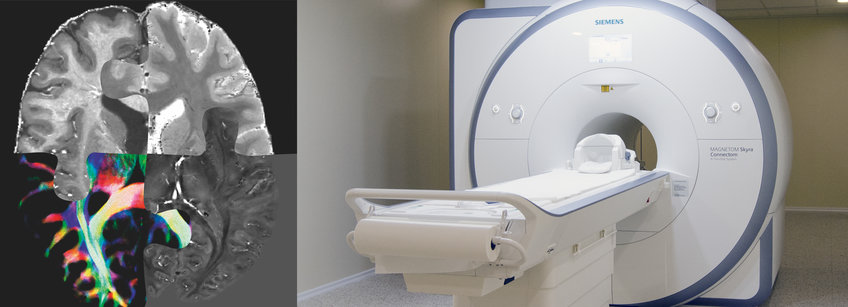
Advanced MRI acquisition: Weiskopf group developed a method that records intrinsically co-localized multiple quantitative parameters maps (MPM) of MR parameters (longitudinal (R1) and effective transverse relaxation rates (R2*), magnetisation transfer (MT), proton density, QSM) with submillimetre resolution (400 µm isotropic) in vivo at 7T using prospective motion correction (PMC). MPMs are sensitive probes of tissue microstructure reflecting brain myelination (R1, R2*, QSM), brain iron accumulation(R2*, QSM) and tissue water content (PD, R1, R2). The ultra-high resolution and unique combination of multiple parameters promises quantitative information about the small nuclei in SN and LC in PD (Fig. 1 A, B). Compared to conventional weighted MRI images, MPMs provide sensitive and specific metrics to follow subtle changes in brain microstructure induced by healthy aging or neurodegeneration in longitudinal studies.
Biophysical models: The group recently further enhanced method specificity by developing and validating advanced biophysical models of MPMs based on quantitative histological information of cellular iron distribution in SN, which quantitatively links MRI parameters to cellular iron distribution. These models relate MPMs, at ultra-high but still mesoscopic resolution, to maps of microscopic distributions of iron-rich dopaminergic neurons in SN, and allows separation of neuromelanin and ferritin iron pools strongly improving MRI specificity. Based on this model, the group proposed a unique quantitative biomarker of dopaminergic cellular density in nigrosome 1 based on combination of R2 and R2* maps, which will be implemented and tested in vivo within IronSleep.
Multimodal atlases: Imaging small subcortical nuclei is challenging due to their small size and complex anatomy. Particularly dopaminergic nigrosomes and the noradrenergic LC are millimetre to submillimetre sized and require the currently maximal MRI resolution in vivo. Therefore, neuroanatomical reference data is required to guide MRI data analysis and interpretation. In collaboration with Dr. Alkemade and her team, Weiskopf group recently contributed to a unique dataset bridging histology and in vivo MRI by combining ultra-high resolution MPMs with 3D multimodal histology on three post-mortem human brains.
IronSleep working plan
Within IronSleep we will implement an MR-based method for in vivo microstructural mapping of brain stem nuclei with submillimeter resolution. For this purpose we will combine advanced ultrahigh resolution MRI acquisition using PMC with the recently developed biophysical models of MR contrast in subcortical nuclei. Informed by the cellular iron distribution from our previous work and anatomical atlases developed in WP2 we will establish optimal imaging protocol for microstructural brain stem mapping. Our method will rely on a novel combination of two biophysical phenomena: i) the effect of neuromelanin-bound iron on the longitudinal relaxation rate (R1) and magnetization transfer (MT) rates of water protons; ii) the distinct contribution of iron-rich dopaminergic cells to R2 and R2* and QSM contrast.
Team – MPI CBS

Professor Dr Nikolaus Weiskopf
Director
Dr Evgeniya Kirilina
Research group leaderDr Kerrin Pine
Scientific researcher
Malte Brammerloh
Doctoral researcher